ENKASTIM™ is a targeted autologous NK cell therapy that activates the patient’s own NK cells to target cancers expressing membrane Hsp70 (“mHsp70”), a cancer-specific biomarker which is highly expressed on aggressive cancer cells, but not on non-cancerous cells.
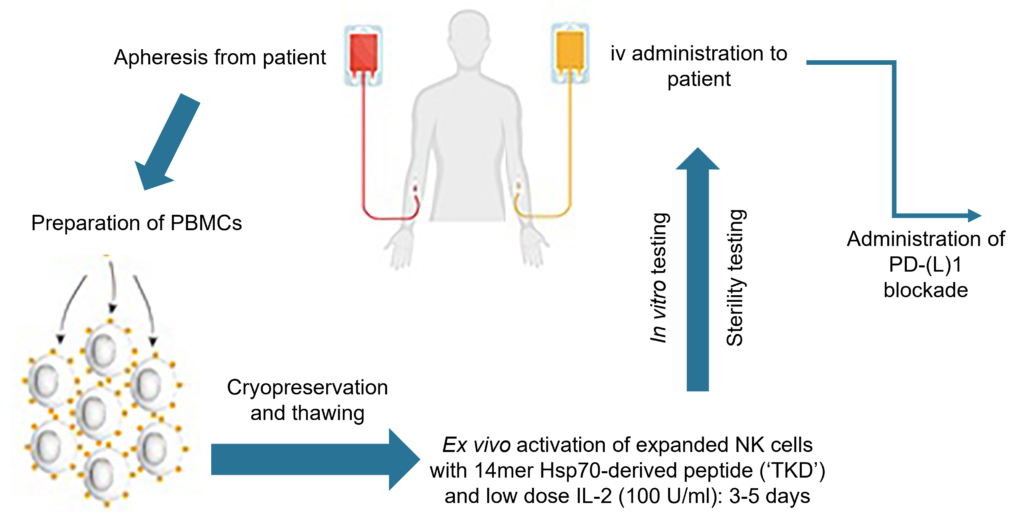
ENKASTIM™ is manufactured by obtaining a patient’s peripheral blood mononuclear cells (“PBMCs”) by leukapheresis in an out-patient setting, followed by ex vivo activation using a patented Hsp70-derived synthetic peptide (named “TKD”) and low-dose interleukin-2 (“IL-2”) before being given back to the patient on four occasions. No genetic modification of the targeted NK cell product is required.
ENKASTIM™ has been evaluated in two clinical trials in Germany.
Completed Phase I clinical trial
Involved twelve (12) patients in Germany, eleven (11) with metastatic colorectal cancer and one (1) with non small lung cancer (NSCLC) receiving ENKASTIM™ after standard of care (SOC). The therapy showed it was safe, well tolerated and had signs of efficacy.
Completed Phase IIa (randomized, controlled) clinical trial
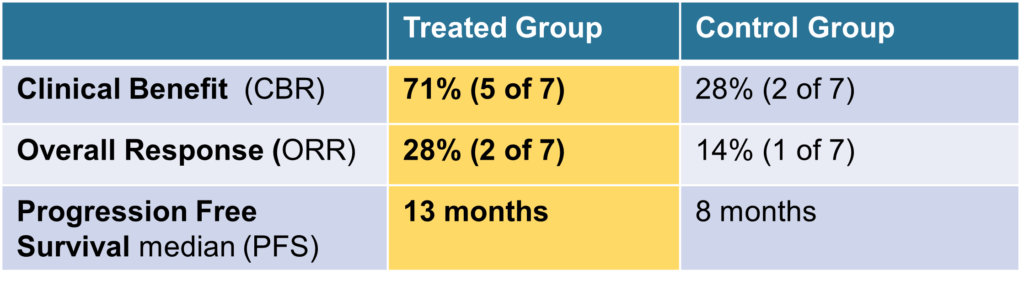
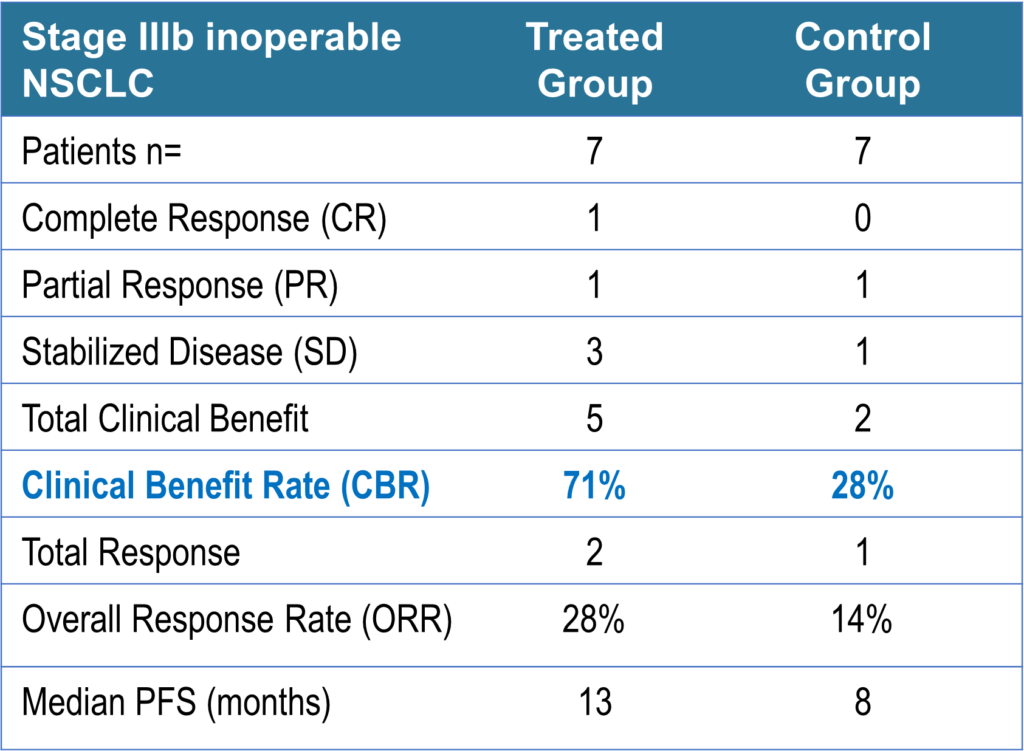
Involved fourteen (14) patients with advanced (Stage IIIb) NSCLC in Germany. Of the seven (7) patients who received ENKASTIM™ after radio-chemotherapy, five (5) patients experienced a clinical benefit (71%), including one complete response (CR) and one partial response (PR). The median Progression Free Survival (PFS) was 13 months, including one patient who left the trial with a PFS of 15 months.
In contrast, of the seven (7) patients who received standard of care radio-chemotherapy alone, only two had a clinical benefit (CBR 28%) and a PFS of 8 months. No patient had a CR.
Planned Phase I/IIa Clinical Combination trial
Patients with advanced (Stage IV) NSCLC will receive ENKASTIM™ plus PD-(L)1 immune checkpoint inhibitor ‘standard of care’. If the clinical trial shows the therapy is effective, a Pivotal Phase IIb trial will be planned for accelerated approval.
1 Krause SW, Gastpar R, Andreesen R, Gross C, Ullrich H, Thonigs G, et al. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: A clinical phase I trial. Clin Cancer Res (2004) 10:3699-3707.
2 Multhoff G, Seier S, Stangl S, Sievert W, Shevtsov M, Werner C, et al. Targeted natural killer cell based adoptive immunotherapy for the treatment of patients with NSCLC after radiochemotherapy – a randomized phase II clinical study. Clinical Cancer Research (2020) 26:5368-5379. doi:10.1158/1078-0432.CCR-20-1141
3 Kokowski K, Stangl S, Seier S, Hildebrandt M, Vaupel P, Multhoff G. Radiochemotherapy combined with NK cell transfer followed by second-line PD-1 inhibition in a patient with NSCLC stage IIIb inducing long-term tumor control: a case study. Strahlenther Onkol (2019) 195(4):352-361. doi: 10.1007/s00066-019-01434-9.
