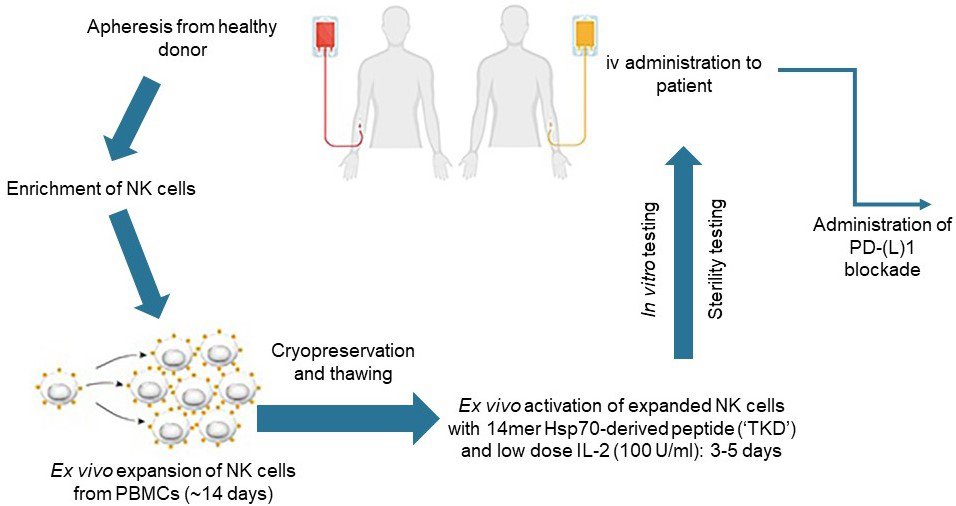
AP-161™ is an allogeneic targeted NK cell therapy that targets tumors expressing mHsp70. For this, cryopreserved NK cells expanded from peripheral blood mononuclear cells (PBMCs) obtained from healthy donors are thawed and incubated ex vivo with TKD and IL-2, after which they are intravenously administered to patients. No genetic modification of the targeted NK cell product is required.
Pre-clinical (animal) studies in Germany have demonstrated that TKD/IL-2 activated human allogeneic NK cells control the growth of human metastatic colorectal cancer, NSCLC, and metastatic pancreatic cancer in immune deficient mice. Pre-clinical studies in immune deficient mice have also shown the efficacy of AP-161™ against human metastatic lung cancer to be further improved when combined with a PD-1 inhibitor.
Planned Phase I clinical combination trial in patients with metastatic gastric and colorectal cancer (Stage IV) in the United States, after standard of care (SOC) combined with PD-(L)1 checkpoint inhibitor patients, who failed chemo-radiation and PD-(L)1 checkpoint inhibitors as salvage therapy.
