EDx700™ Liquid Biopsy
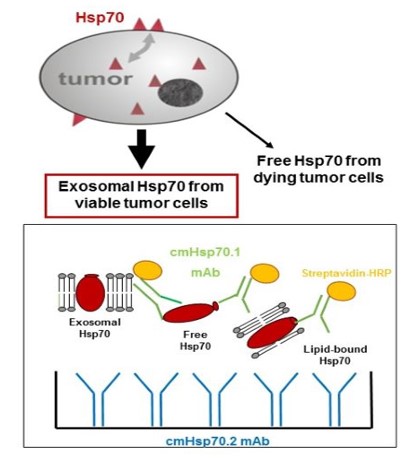
EDx700™ liquid biopsy is a simple blood test which measures exosome-associated Hsp70 in the blood – no tissue biopsy is needed.
EDx700™ detects the presence of tumors expressing mHsp70, and can be used to measure therapeutic response and monitor early disease recurrence.
Clinical Trial – Companion Diagnostic Was approved to be used as part of the inclusion/exclusion criteria for a Phase IIa clinical trial of our autologous membrane Hsp70 targeted NK cell therapy in patients with Stage IIIb NSCLC by the Paul Ehrlich Institute (‘German FDA’) .
To be utilized in a Phase I/II Clinical Trial in patients with advanced Stage IV NSCLC in the United States.
EDx700™ detects the presence of tumors expressing mHsp70, and can be used to measure therapeutic response and monitor early disease recurrence.
Clinical Trial – Companion Diagnostic Was approved to be used as part of the inclusion/exclusion criteria for a Phase IIa clinical trial of our autologous membrane Hsp70 targeted NK cell therapy in patients with Stage IIIb NSCLC by the Paul Ehrlich Institute (‘German FDA’) .
To be utilized in a Phase I/II Clinical Trial in patients with advanced Stage IV NSCLC in the United States.
Commercialization
Alphageneron has entered into a royalty agreement for EDx700™ with Exocellular Diagnostics, an affiliated company.
The test is approved for marketing as a Laboratory Diagnostic Test (LDT) in the European Union, and marketing approval as an LDT in the USA is being sought.
The test is approved for marketing as a Laboratory Diagnostic Test (LDT) in the European Union, and marketing approval as an LDT in the USA is being sought.
Breuninger S et al., Quantitative analysis of liposomal heat shock protein 70 (Hsp70) in the blood of tumor patients using a novel lipHsp70 ELISA. J Clin Cell Immunol 2014, 5.5.
Gunther S et al., Correlation of Hsp70 serum levels with gross tumor volume and composition of lymphocyte subpopulations in patients with squamous cell and adeno NSCLC. Front Immunol 2015, 6:556.
Werner C et al., Hsp70 in liquid biopsies – A tumor-specific biomarker for detection and response monitoring in Cancer. Cancers 2021, 13, 3706.
Seier S et al., Elevated levels of circulating Hsp70 and an increased prevalence of CD94+/CD69+ NK cells is predictive for advanced stage non-small cell lung cancer. Cancers 2022, 14:5701.
